About NUCBTR
NUCBPI is a strategic network of infrastructures for fundamental and translational biomedical research, which includes partners from two of the largest medical universities in Bulgaria (MU-Sofia and MU-Plovdiv). Moreover, it includes a number of hospitals and research centers. In parallel, in the infrastructure some of the largest biobanks for genetic and tissue material in the country are comprised.
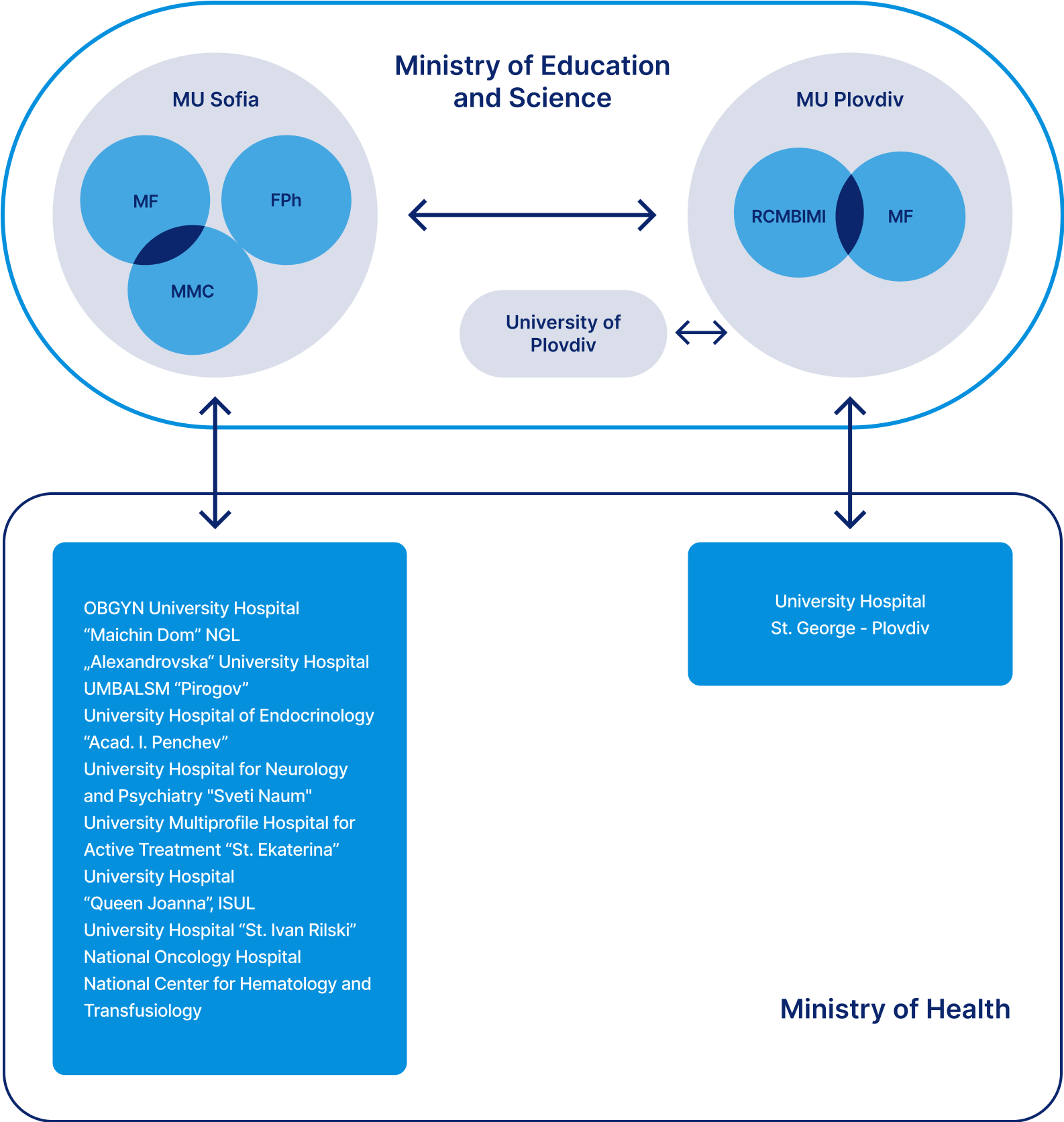

Organizations - members of the consortium:
Medical University - Sofia (MU - Sofia)
MU - Sofia is the oldest institution for higher medical education. It is a leading national educational, scientific and medical institution that pursues an educational and health policy that meets world standards as well as the Bulgarian Medical Academy. MU - Sofia is a complex structure composed of four faculties, one department, three colleges and fourteen university hospitals. It employs 1,459 lecturers and research associates, trains nearly 10,850 Bulgarian and foreign students, doctoral students and postgraduates. MU - Sofia is a well-functioning democratic decentralized structure, with an overall score of 9.56 out of 10 possible in the last institutional accreditation of universities from 19.12.2019.
Members from Medical University - Sofia:
Molecular Medicine Center
The Molecular Medicine Center, Department of Medical Chemistry and Biochemistry (DMCB), Faculty of Medicine is a leading national high-tech research center for biomedical research and the basis of NUCBTR. The laboratories of Metabolomics and Cell Signaling of the Department of Medical Chemistry and Biochemistry also participate. In addition to the DMCB, the Medical Faculty also comprises the Department of Physiology and Clinical Laboratory and Clinical Pharmacology (CLCP) of the University Hospital "Alexandrovska".
Medical Faculty
Pharmaceutical Faculty
Associated partners:
Medical University - Plovdiv (MU - Plovdiv)
MU - Plovdiv is among the leading universities accredited by the National Accreditation Agency with a total score of 9.40 out of 10 possible for training students in medicine, dentistry, pharmacy and bachelor's degrees in the regulated professions in the fields of health and health care. It is made up of four faculties, one department, one college, 6 university hospitals and a research institute. More than 5,800 Bulgarian and foreign students, doctoral students and postgraduates study there.
Participants from the Medical University - Plovdiv:
Medical Faculty
Pharmaceutical Faculty
Research Center of Immunology (RCI)
Molecular Medicine and Pharmacogenetics Center
Associated partners:
УМБАЛ ”Св. Георги”, Пловдив
Associated partners:
and over 10 universities and research institutes working in the biomedical field.
Infrastructure management framework
The coordinator of the project for creation of the infrastructure is Acad. Prof. Dr. Vanyo Mitev, PhD, Head of the Department of Medical Chemistry and Biochemistry, First Deputy Rector of the Medical University - Sofia from 2007 to 2016 and Administrative Director of the Molecular Medicine Center since 2008.
The management of the infrastructure is the main responsibility of the Project Coordinator and of two management structures with clearly defined responsibilities, acting together and in coordination: the Management Board (MB) and the Scientific Board (SB). The Board will ensure the quality and timely implementation of the objectives of the University complex under the supervision of the Supervisory Board, composed of prominent scientists from Bulgarian and international research institutions independent of the Medical University - Sofia. The Scientific Board meets once a year and is responsible for monitoring the implementation of research projects and determining guidelines for future work of the University complex. The National Assembly coordinates its efforts with those of the faculties and the Academic Councils of the Medical Universities building the national road infrastructure in order to observe the general scientific goals of NUCBTR. The Board is responsible for the daily work of the University complex in close coordination with the administrative and financial departments of the two medical universities. His team meets once a month and acts in accordance with the decisions of the National Assembly, taking responsibility for the evaluation and implementation of scientific products.
The Supervisory Board consists of a team of external experts who monitor the work and provide an independent assessment of the directions and effectiveness of NUCBTR development. The Supervisory Board prepares a report consisting with an assessment and recommendations on the scientific achievements of the research groups, the quality of their work, as well as future guidelines for the development of the research capacity. Furthermore, it brings it to the attention of the Coordinator and the governing bodies of the University complex.

